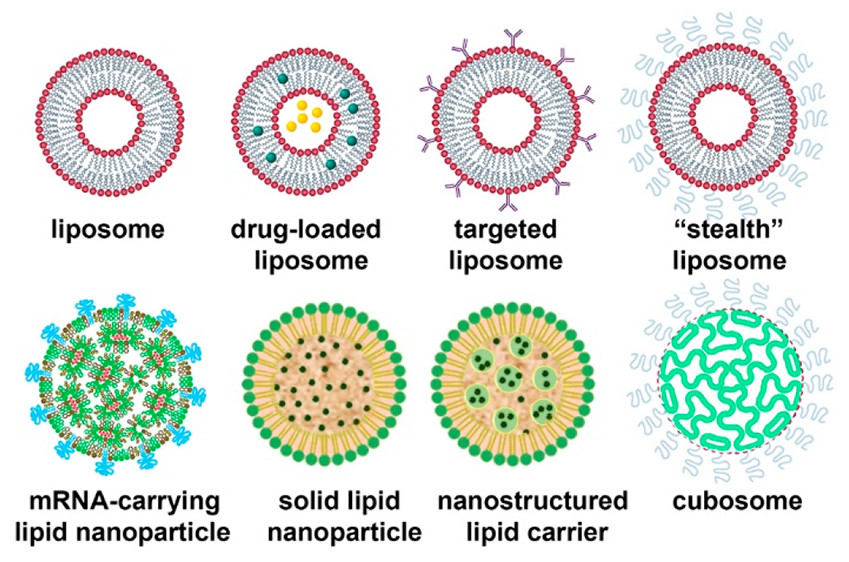
Lipid nanoparticle (LNP) protect mRNA from degradation and facilitate delivery into target cells. They are a versatile formulation technology in many applications.
The properties of LNPs must be tailored according to their specific biomedical application.
Lipid Cleaning
A key challenge in designing lipid nanoparticle formulations is cleaning the lipids to ensure the resulting particles are free from any remaining drug. This is accomplished using various techniques, including high-pressure homogenization (HPH), microfluidic chip mixing, sonication, and freeze-thaw.
Another critical consideration in lipid nanoparticle formulation is the phase transition temperature, which can influence the lipid composition and formulation process. Lipids can form liposomes at temperatures above the phase transition temperature, where hydrophobic tails of phospholipids can be oriented to form a continuous bilayer membrane.
Lipid nanoparticles are versatile, low-viscosity carrier systems that formulate pharmaceuticals, vaccines, nutraceuticals, and diagnostics for topical, pulmonary, oral, and parenteral delivery. Their unique properties and biocompatibility have made them an attractive alternative to other carrier systems for poorly water-soluble drugs. However, several challenges remain in the development and commercialization of lipid-based nanoparticles. In particular, these challenges include optimizing lipid compositions and formulation processes to produce stable and biocompatible particles with desirable encapsulation efficiency and in vivo activity.
Lipid Composition
Lipids are oily, nonpolar organic molecules that are soluble in nonpolar solvents but insoluble in water (a polar molecule). They are found throughout the body and serve various functions, including energy storage, hormone production, and cell membrane architecture.
The most abundant lipids in biological membranes are phospholipids, which contain a polar head group and two hydrophobic hydrocarbon tails. The fatty acid carbon chains comprising the tails can vary in length and saturation (saturation refers to how many carbon atoms are unsaturated). Differences in these properties alter a lipid bilayer’s fluidity, influencing its permeability to small water-soluble molecules.
Phospholipids may be formed as spherical micelles, where the hydrophobic tails are buried within the center, or as bimolecular sheets, where the tails extend outward to include two faces of a symmetrical tetrahedron shape. Hydrogen bonds then link the tetrahedrons to form a continuous, fluid bilayer.
Lipids can also change their charge in response to the environment, an essential property for lipid nanoparticles. For example, ionizable lipids become protonated at low pH, giving them a positive charge. This allows lipids to interact less strongly with the anionic mRNA inside blood cells and improves the encapsulation efficiency of LNPs.
Lipid Structure
Lipids are a large class of molecular building blocks that serve as energy-storage molecules, chemical messengers, and structural components of cells. They are characterized by their amphiphilic nature, with polar heads and nonpolar tails. Fatty acids, phospholipids, and cholesterol are examples of lipids. Other lipids include sterols, which have four fused carbon rings, and isoprenoids, based on a five-carbon branched chain of isomers, such as the profoundly colored lycopene in tomatoes and carotenoids in autumn leaves.
Many glycerophospholipids, such as phosphatidylcholine (PC) and sphingomyelin, can form bilayers in water. When these molecules are assembled into a lipid nanoparticle, they can be stabilized using the self-association mechanism of amphipathic molecules, which is based on a packing theory that relates the molecule’s shape to its geometry when assembled into a larger structure.
Lipid nanoparticles are usually spherical, ranging from 10 to 1,000 nanometers. They can be formulated with ionizable lipids to deliver nucleic acids such as siRNA or mRNA, which are then used to deliver therapeutics into cells through the invagination of the cell membrane.
Lipid Biodistribution
The composition of lipids and their biodistribution can influence the encapsulation efficiency, intracellular uptake, and delivery efficacy of LNPs. LNPs usually comprise a core of phospholipids (DSPC, DOPE, or DPPE) and helper lipids, such as cholesterol and cholesteryl oleate. Cholesterol is a lipid that can enhance the stability of LNPs, preventing them from aggregation and improving the permeability of the liposomal membrane for cytosolic entry and lipid partitioning.
The cholesterol derivative’s molecular geometry can influence lipid nanoparticle encapsulation and cell-specific delivery.
Different lipid structures can also be used to achieve organ-specific or cell-specific delivery. In addition, the distribution of lipids can be determined using DNA barcode oligonucleotides to perform in vivo high-throughput characterization of lipid distribution at the cell level.
Lipid Stability
The stability of lipids is an essential consideration in a wide range of applications. Lipid nanoparticles (LNPs) undergoing freezing and thawing cycles or long-term storage may suffer from significant enzymatic degradation that affects particle concentration. This degradation often occurs during the small intervals between thawing and extraction, resulting in low-volume, subvisible particle measurements.
Backgrounded Membrane Imaging (BMI) offers a superior solution to these challenges, delivering high-throughput and accurate subvisible particle analysis of complex, liquid lipid formulations.
The stability of bolalipid-monopolar lipid mixed layers depends on the packing of the hydrophobic monopolar lipid hydrocarbon chains and the membrane-spanning bolalipid chains, which form a bilayer structure. The molecular theory suggests that the bilayer is stable at bolalipid concentrations close to zero and one, the minimum concentrations necessary for creating the polar lipid-water interface in the phase separation. The bilayer permeability is also reduced with bolalipids due to the shorter transmembrane chains. The bolalipid bilayer is less susceptible to delamination, a common occurrence in lipid membranes.